Gabriele Coli MSc
Leonard S. Ornstein Laboratory, room 0.14
Princetonplein 1, 3584 CC Utrecht
P.O. Box 80 000, 3508 TA Utrecht
The Netherlands
phone: +31 (0)30 253 2831
secretariat: +31 (0)30 253 2952
e-mail: g.m.coli@uu.nl
Research
Promotor: Prof. dr. ir. Marjolein Dijkstra
Funding: NWO
Employed: 1 November 2017 – 31 October 2021
Simulations of nucleation of colloidal Laves phases
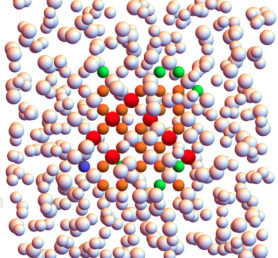
Colloidal crystals with lattice spacings similar to the wavelength of light are perfectly suitable for the manipulation of light itself. The currently known clear champion structures to realize photonic crystals are the colloidal analogues of the MgCu2, MgZn2 and MgNi2 Laves phases [1]. The huge variety of potential applications, ranging from photovoltaic cells to optical computer chips, makes it fundamental to understand how to tune the parameters to favor the formation of a crystal structure with respect to another. To this aim, we investigate the nucleation process of the Laves phases by means of numerical simulation. In order to control the kinetic self-assembly process, the size, structure and rate with which a nucleus appears are crucial parameters. To probe these rare events we implement the seeding approach [2], which consists in inserting a crystal cluster of a known shape and size into a metastable fluid configuration. Keeping the temperature fixed and running the simulations for different values of the pressure we calculate the critical size of the crystal cluster which is stable at that pressure value. This information, together with other physical observables of interest – e.g. difference of chemical potential between the two phases, kinetic pre-factor of the nucleation rate and density of the metastable fluid – gives a full description of the process, including the value of the Gibbs Free Energy barrier to overcome in order to complete the transition. It is important to stress that this method strongly relies on the validity of Classical Nucleation Theory. To distinguish crystal particles from fluid particles we use bond orientational order parameters based on the computation of spherical harmonics and we then identify the nuclei using a clustering algorithm [3].
Figure 1: Crystal cluster of MgCu2 inserted in a fluid configuration of mixture of nearly-hard spheres. The particles are coloured as follows: i) Red: Large MgCu2-like particles ii) Orange: Small MgCu2-like particles iii) Pink: fluid-like particles iv) Other colours: different unidentified structures.
[1] A.P. Hynninen et al., Nature Materials 6, 202-205 (2007)
[2] J. R. Espinosa et al., The Journal of Chemical Physics 144, 034501 (2016)
[3] L. Filion et al., The Journal of Chemical Physics 133, 244115 (2010)