Jessi van der Hoeven
Leonard S. Ornstein Laboratory, room 0.12 / David de Wied building 4th floor
Princetonplein 1, 3584 CC Utrecht
P.O. Box 80 000, 3508 TA Utrecht
The Netherlands
phone: +31 (0)30 253 2925
secretariat: +31 (0)30 253 2952
e-mail: j.e.s.vanderhoeven@uu.nl
Research
Promotors: Prof. dr. Petra de Jongh and Prof. dr. Alfons van Blaaderen
Funding: NWO-Vici, EU-ERC Advanced, Debye Graduate Program
Employed: 1 December 2014 – 30 November 2018
In Situ Alloying of Bimetallic Nanorods
Bimetallic Au-Ag nanoparticles receive much attention for their application in gas-phase catalysis. Generally, the catalytic properties of such bimetallic nanoparticles critically depend on the distribution of the two metals, and their interaction with a stabilizing oxide. However, so far it has been a great challenge to characterize the evolution of the atomic distribution within the particles under realistic conditions and access the parameters that influence the atomic redistribution.
In this project, we study the alloying of Au-Ag based nanoparticles as a function of the nanoparticles’ metal-to-metal ratio and the surrounding gas atmosphere during heat treatment. As a model system we use single crystalline Au-core Ag-shell nanorods embedded in a mesoporous silica of which the shape, size and stoichiometry can precisely be controlled by careful colloidal growth of the gold nanorods, followed by deposition of Ag shells of a given thickness [1-2].
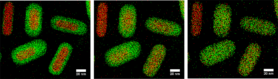
For this well-defined model system we study the influence of the metal-to-metal ratio and gas atmosphere (reducing, oxidizing, inert) on the mixing of the the Au-core with the Ag-shell in situ. To this end, we follow the alloying process at a single particle level with in situ heating TEM measurements in high vacuum (Figure 1), on an atomic level with in-situ EXAFS measurements in inert, oxidizing and reducing gas atmospheres, with ex situ oven measurements in absence of an electron or x-ray beam and under catalytic conditions in the selective hydrogenation of butadiene.
Figure 1 – EDX maps showing the transition of mesoporous silica coated Au-core (red) Ag-shell (green) nanorods (left) to alloyed Au-Ag nanorods (right). Images were acquired by performing in-situ heating TEM. [1] T.S. Deng, et al., Chem. Mater. 27, 7196-7203 (2015)
[2] W.A. Albrecht et al., Nanoscale, 9, 2845-2851 (2017)