Kelly Brouwer MSc
Leonard S. Ornstein Laboratory, room 0.07
Princetonplein 1, 3584 CC Utrecht
P.O. Box 80 000, 3508 TA Utrecht
The Netherlands
phone: +31 (0)30 253 2831
secretariat: +31 (0)30 253 2952
e-mail: j.h.brouwer@uu.nl
Research
Promotor: Prof. dr. Alfons van Blaaderen
Project: “Fundamentals of catalysis: Ultimate control over nanoparticle structure, composition, size, and location in supported bimetallic catalysts”
Binary supraparticles: using self-assembly to control the structure in bimetallic catalysts
Self-assembly offers a promising new route in designing heterogeneous catalysts.[1] The main advantage of this self-assembly approach, with respect to conventional preparation methods, is the outstanding control over size, location and distribution of the active (metal) nanoparticles within the self-assembled catalyst particle, so-called supraparticle.
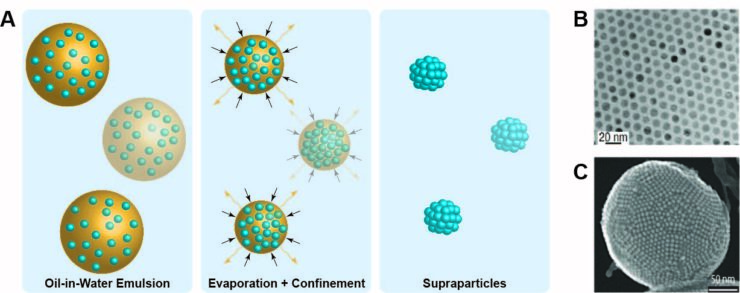
Supraparticles are typically tens of nanometers to a few microns sized particles composed of many nanoparticles, see Figure 1C.[2] In this project the synthesis of binary supraparticles will be investigated using a metal nanoparticle (for example iron, cobalt, nickel, palladium, and bimetallic nanoparticles) as one building block and the other building block being a support material (for example silica). For the nanoparticles to crystallize in well-defined supraparticles, the nanoparticle building blocks need to be highly monodisperse. In the case of binary supraparticles, the size ratio of the two different nanoparticles needs to be chosen properly to allow crystallization.
Crystallization of the nanoparticles into supraparticles is induced by slowly evaporating emulsion droplets containing a nanoparticle dispersion, as depicted in Figure 1A. The spherical confinement induced by the shrinking emulsion droplet then results in well-defined supraparticles. In this project emulsions are prepared via several methods among them shaking, sonication, shear cell emulsification and fluidic methods. Especially with using fluidic devices, monodisperse emulsion droplets can be generated. In this project a fluidics chip will be investigated (in collaboration with the University of Twente). The chip can produce 1 µm emulsion droplets in 5000 nanochannels simultaneously, making it possible to generate grams of highly monodisperse supraparticles in just one night.
Synthesized supraparticles will be analyzed using electron microscopy, electron tomography, and 3D-STED confocal microscopy. The most suitable characterization technique depends on the size and composition of the supraparticles. With help of these characterization techniques, the impact of different particle sizes and size ratio’s on the packing of the individual nanoparticles within the supraparticle can be examined.[3]
Figure 1: A) Schematic of the steps involved in the self-assembly of nanoparticles (individual blue spheres) into supraparticles(blue clusters) through slowly evaporating emulsion droplets. For simplicity ligand molecules are absent in this figure. B) Monodisperse FexO nanoparticles [4]. C) Supraparticle comprised of metal oxide nanoparticles [2].
[1] Y. Kang et al., JACS, 135, 1499-1505, (2013)
[2] B. de Nijs et al., Nature Materials, 14, 56-60, (2014)
[3] D. Wang et al., Nature communications, 9, 2228, (2018)
[4] J. Park et al., Nature Letters, 3, 891-895, (2004)