Dr. Freddy Rabouw
Leonard S. Ornstein Laboratory, room 0.21
Princetonplein 1, 3584 CC Utrecht
P.O. Box 80 000, 3508 TA Utrecht
The Netherlands
phone: +31 (0)30 253 3519
secretariat: +31 (0)30 253 2952
e-mail: f.t.rabouw@uu.nl
employed since January 2017
MCEC funding
Research
Research
I obtained my PhD degree from Utrecht University (2015). Currently (2016–2017) I am a NWO Rubicon fellow at ETH Zurich. Starting in 2017 I have joined the MCEC program, and from 2018 will fully return to Utrecht University. Below you find a list of my main research topics, including a recent highlight:
(1) Optical spectroscopy of (individual) nanoparticles
Recent highlight from F.T. Rabouw, M. Kamp, R.J.A. van Dijk-Moes, D.R. Gamelin, A.F. Koenderink, A. Meijerink & D. Vanmaekelbergh, Nano Lett. 15, 7718–7725 (2015)
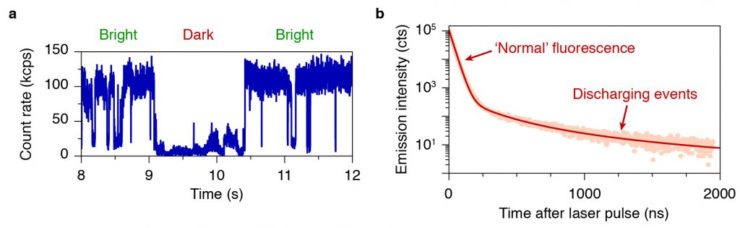
‘Blinking’ is a phenomenon encountered in almost all visible-light emitters such as fluorescent dye molecules or nanocrystals. The fluorescence of an individual emitter randomly switches (i.e. ‘blinks’) between bright and dark, while continuously excited (Fig. 1a). After 20 years of research on this phenomenon, the origin is still unclear. For fluorescent nanocrystals, blinking is most commonly ascribed to random charging (rendering the nanocrystal dark) and discharging (returning it to the bright state). We found that each discharging event releases a photon (Fig. 1b). As a result, the statistics of charging and discharging can be studied using photoluminescence decay measurements (Fig. 1b). We discovered that charging and discharging occurs much more frequently than assumed by existing models.
(2) Modeling of charge-carrier and excited-state dynamics
Recent highlight from A.C. Berends, F.T. Rabouw, F.C.M. Spoor, E. Bladt, F.C. Grozema, A.J. Houtepen, L.D.A. Siebbeles & C. de Mello Donegá, J. Phys. Chem. Lett. 7, 3503–3509 (2016)
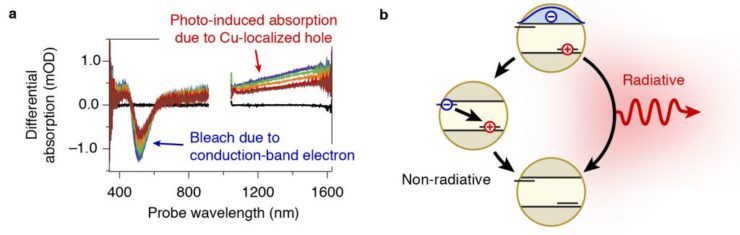
The best-developed fluorescent nanocrystals in terms of color-control and brightness are based on cadmium, which leads to toxicity concerns for applications. Luminescent CuInS2 nanocrystals offer a cadmium-free alternative. However, the brightness of these nanocrystals is not yet optimal. We studied the charge-carrier dynamics in CuInS2 nanocrystals to identify the origin of energy losses. Excited electrons and holes show distinct spectral signatures in transient-absorption measurements (Fig. 2a). We found that luminescence originates from holes after they localize on a Cu-atom. Losses arise when electrons are trapped (presumably on the nanocrystal surface) and then recombine with a hole non-radiatively (Fig. 2b). For improved CuInS2 nanocrystals electron traps should be avoided, for example by growing a protective shell.
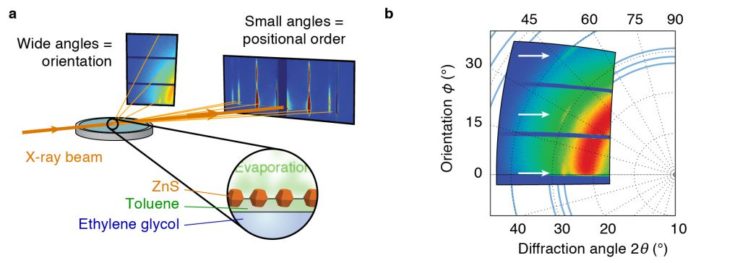
(3) Self-organization of nanoparticles into larger (ordered) superstructures
Recent highlight from W. van der Stam, F.T. Rabouw, S.J.W. Vonk, J.J. Geuchies, H. Ligthart, A.V. Petukhov & C. de Mello Donegá, Nano Lett. 16, 2608–2614 (2016)
Nanocrystals can be used as building blocks for ‘metamaterials’, like atoms are the building blocks of regular materials. In general, the properties of a material depend on the type of building blocks and on the arrangement. In contrast to atoms, nanocrystals can have an anisotropic shape. The properties of these (e.g. luminescence polarization) can additionally depend on orientation. We studied the self-assembly of anisotropic bifrustum-shaped ZnS nanocrystals into an ordered 2-dimensional metamaterial, by controlled evaporation of a dispersion in toluene. Using grazing-incidence x-ray scattering (Fig. 3a), we could simultaneously follow the positional order of the nanocrystal (at small scattering angle) and the orientational order (at wide angle). We found that the addition of oleic acid to the system caused the nanocrystals to align their crystallographic c-axis with respect to each other (Fig. 3b). This effect is probably caused by selective adsorption of oleic acid on specific crystal facets.