Dr. Murphy (Wun-Fan) Li
Ornstein Laboratory, room 1.73
Princetonplein 1, 3584 CC Utrecht
P.O. Box 80 000, 3508 TA Utrecht
The Netherlands
phone: +31(0)30 253 3125
secretariat: +31(0)30 253 2952
e-mail: w.f.li@uu.nl
Research
Supervisor: Dr.ir. M.A. van Huis
Promotoren: Prof.dr.ir. Marjolein Dijkstra and Prof.dr. A. van Blaaderen
Employed s PhD student from November 2012 till May 2017
Funded by NWO
Employed as postdoc till 28 Feb 2018
Ab-initio calculations (atomistic approaches), Density functional theory (DFT), Transmission electron microscopy (TEM)
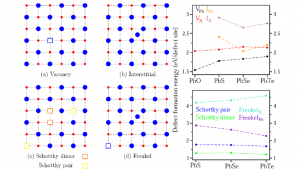
Vacancy, interstitial, Schottky, and Frenkel defects in rock salt (RS) phase lead chalcogenide systems PbS, PbSe, and PbTe (PbX) were studied systematically using the first principles Density Functional Theory (DFT). PbO in the RS phase was also included for comparison. The studied physical properties include defect formation energy, local geometry relaxation, Bader charge analysis, and electronic structure. The current results of defect formation energies indicate that the monovacancy defects and Schottky defects are more favored. The type of defect with lowest defect formation energy is Schottky dimer. The calculated values are 1.27 eV, 1.29 eV, and 1.21 eV for PbS, PbSe, and PbTe, respectively. Thus monovacancies and Schottky defects may play an important role in the cation exchange process of heterogeneous nanocrystals (HNCs)[1]. Charge analysis indicates electride behavior at anion vacancy sites, where the charge density is accumulated. The applicability of a Coulomb model to describe the energetics of inter-defect separation in Schottky configurations are investigated. The calculated Coulomb energy of 0.015 eV, being much smaller than the defect formation energy change (0.5 eV to 1.0 eV), shows that the Coulomb model is not viable in this case. The dependence of defect formation energy on inter-defect distance could be attributed to lattice relaxation.
Figure 1: 2D Schematic of the models (left). Pb atoms in red, and X atoms in blue. The calculated formation energy for different defect types (right).
[1] A. O. Yalcin, Z. Fan, B. Goris, W.-F. Li, R. S. Koster, C.-M. Fang, A. van Blaaderen, M. Casavola, F. D. Tichelaar, S. Bals, G. V. Tendeloo, T. J. H. Vlugt, D. Vanmaekelbergh, H. W. Zandbergen, and M. A. van Huis, Nano Lett. 14, 3661 (2014)